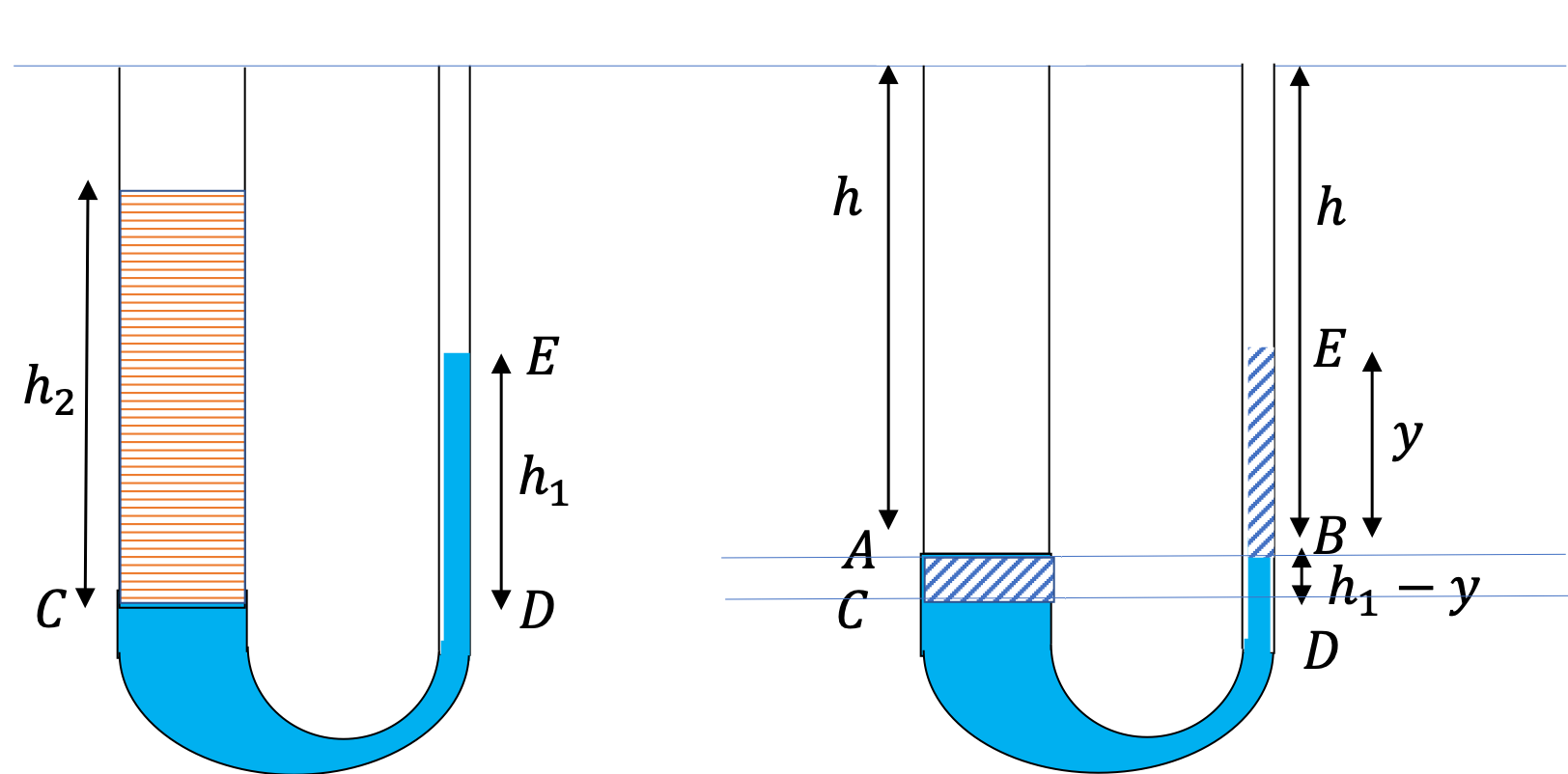
Example 17.13. Fluid Heights in an Open U-tube.
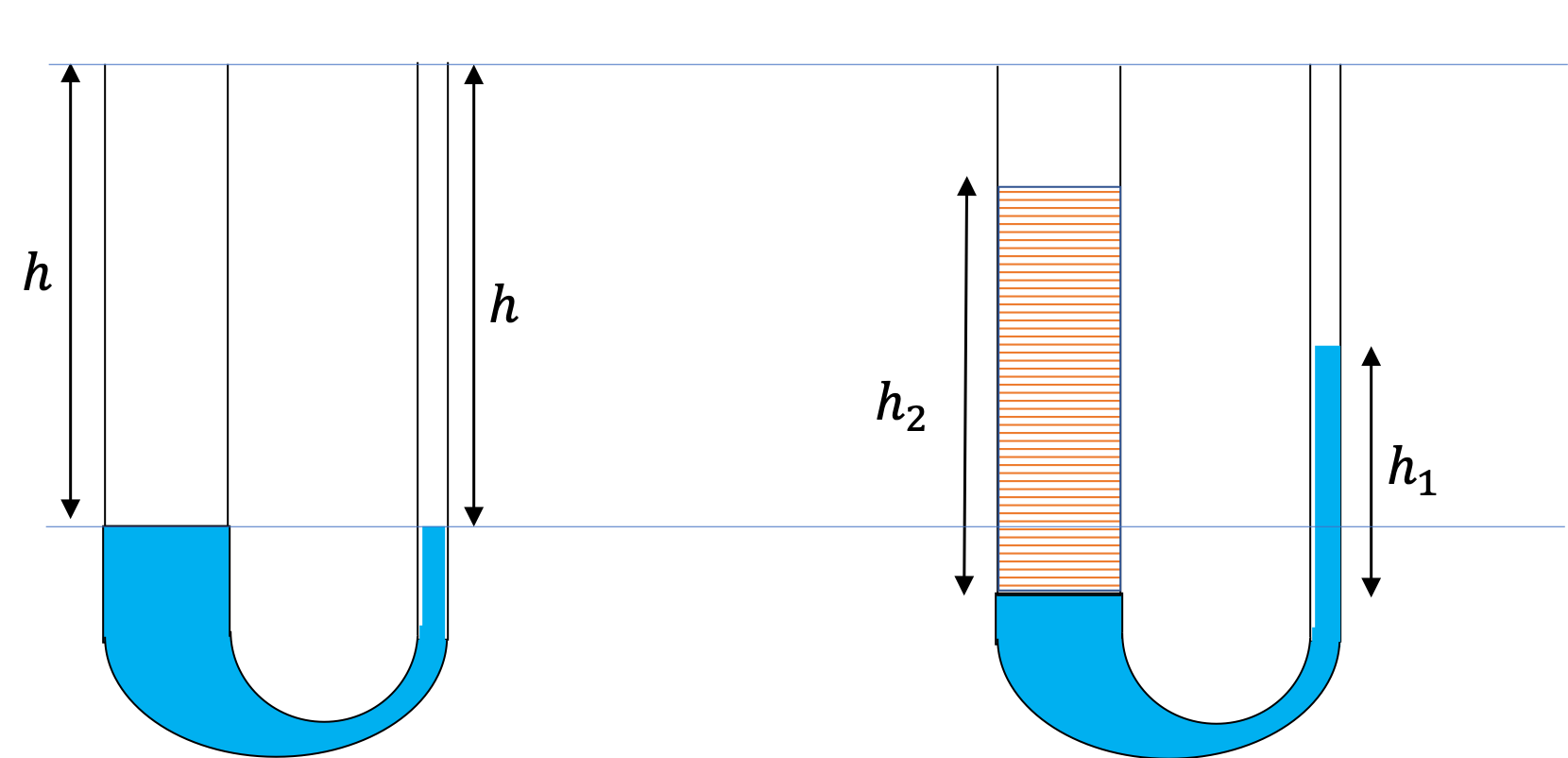
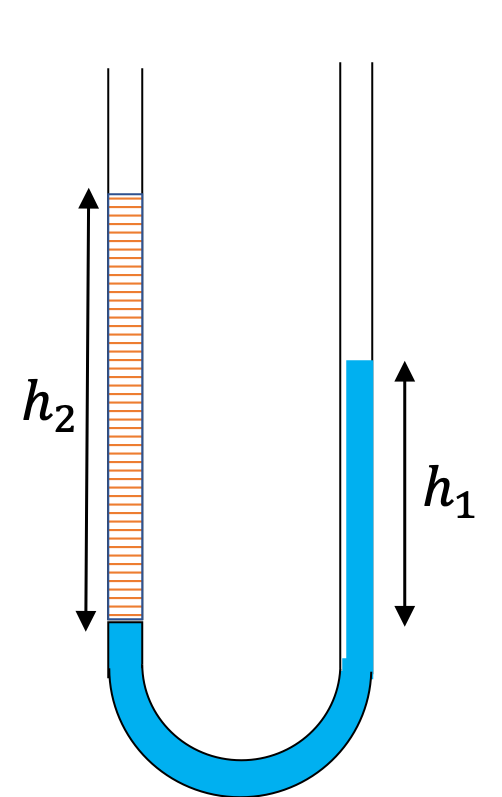
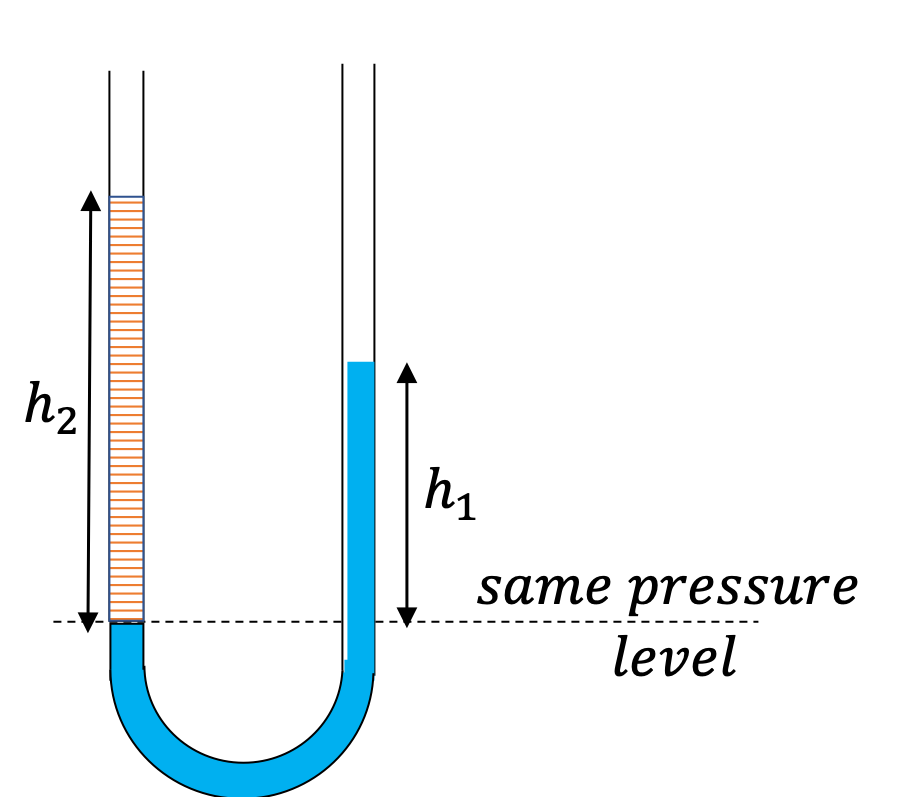
A U-tube with both ends open is filled with a liquid of density \(\rho_1\) to a height \(h \) on both sides. A liquid of density \(\rho_2\lt \rho_1\) is poured on one side. We find that liquid 2 settles on top of liquid 1, with the heights on the two sides now becoming different. The height to the top of the liquid 2 from the interface of the two liquids is \(h_2\) and the height to the top of liquid from the level of the interface is \(h_1\text{.}\) Find \(h_2 - h_1 \text{.}\)
Answer.
\(\left( 1- \frac{\rho_1}{\rho_2} \right) h_2\text{.}\)
Solution.
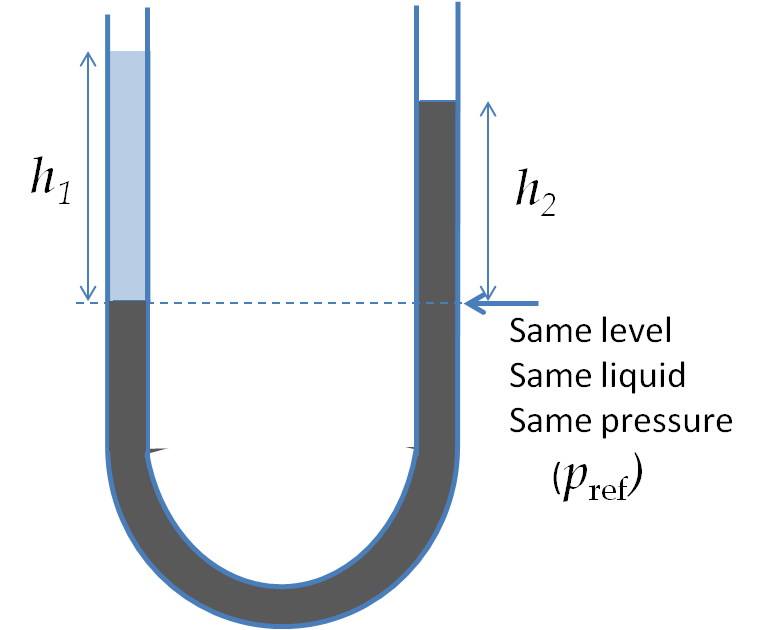
The pressure at the same height on the two sides of a U-tube must be same as long as the two points are connected by a continuous line in the same liquid. Therefore, we consider two points at the same level in the two arms of the tube: One point will be the interface on the side of the liquid 2 and the other will be a point in the arm with liquid 1 that is at the same level as the interface in the other arm.
\begin{align*}
\amp \text{Pressure on the side with liquid 1} = p_0+\rho_1 g h_1. \\
\amp
\text{Pressure on the side with liquid 2} = p_0+\rho_2 g h_2.
\end{align*}
Since, the two points are in liquids 1, one at the top of the left side of liquid 1 and the other in the liquid on the right side at the same height, the pressure at the two points must be the same pressure. Therefore, we have
\begin{equation*}
p_0+\rho_1 g h_1 = p_0+\rho_2 g h_2.
\end{equation*}
Hence,
\begin{equation*}
\rho_1 h_1 = \rho_2 h_2.
\end{equation*}
This says that the difference in heights of the two sides on the U-tube would be
\begin{equation*}
h_2-h_1 = \left( 1- \frac{\rho_1}{\rho_2} \right) h_2.
\end{equation*}
The result makes sense if we set \(\rho_2=\rho_1\text{,}\) which gives \(h_2=h_1\text{.}\) If the two sides have the same density, they would have the same height.